2020 EHR Reporting Requirements (EH/CAH)
An overview of the US Medicare Promoting Interoperability Program EHR reporting requirements for CY 2020.

The US Centers for Medicare & Medicaid Services is working to improve the meaningful use of certified electronic health record (EHR) technology through Promoting Interoperability Programs. (Formerly known as Meaningful Use Programs.) Each year, health care organizations eligible for Medicare payments in the US must report on a defined set of requirements. For the calendar year 2020, the requirements for Eligible Hospitals (EHs) and Critical Access Hospitals (CAHs) are summarized below.
Note: This post only represents a summary of the requirements and should not be used as your information source for submissions to CMS. For detailed information on all components, you must refer to official documentation (CMS.gov)
Summary of the 2020 EHR Medicare Promoting Interoperability Reporting Requirements

2015 Edition CEHRT
For Medicare Promoting Interoperability Program 2020 Reporting, organizations are required to use 2015 Edition CEHRT technology.
It is not necessary to have had this in place as of Jan 1, 2020. The 2015 CEHRT EHR must be in place for the start of the selected reporting period. It must remain in use for the duration of the organization reporting timeframe.
Score 50 or Greater on Objective Measures
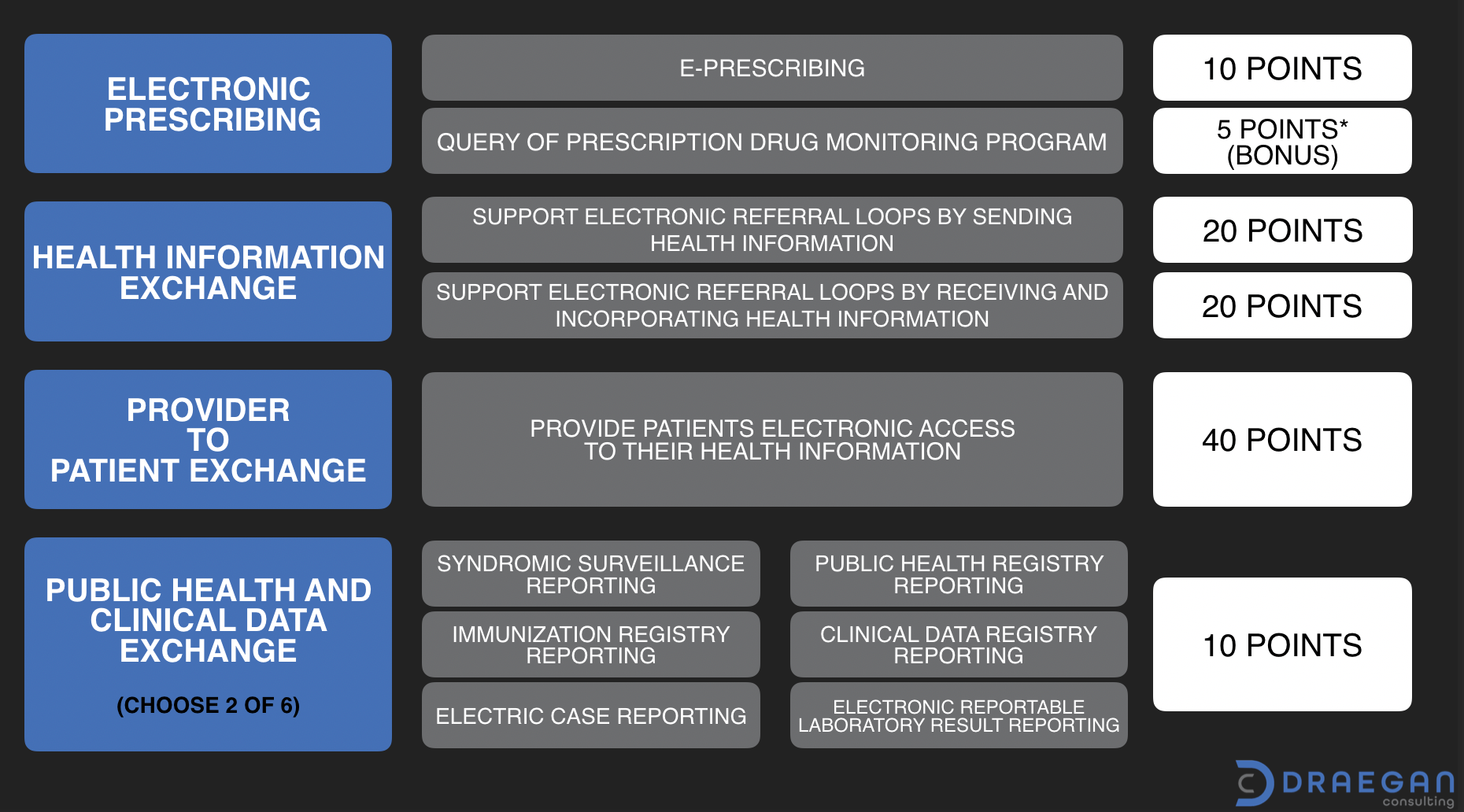
There are four (4) objectives associated with EH/CAH 2020 reporting. Scoring associated with objective measures is performance-based. To meet the reporting criteria, organizations need to achieve a score of 50 or greater out of 100.
Electronic Prescribing (10 points + option for 5 bonus)
There are two measures associated with this objective. The first is the ePrescribing measure worth a total of 10 points. It requires numerator/denominator reporting. The second measure is optional for the 2020 year and can provide 5 bonus points. It is an attestation only measure for Query of the Prescription Drug Monitoring Program (PDMP).
Health Information Exchange (40 points)
The Health Information Exchange objective is worth a total of 40 points split evenly between two (2) measures.
- Support Electronic Referral Loops by Sending Health Information
- Support Electronic Referral Loops by Receiving and Incorporating Health Information
These measures support information exchange during transitions of care. For the sending measure, a Summary of Care record needs to be created and electronically sent. The receiving and incorporating measure, focuses on reconciliation. Inbound medications, medication allergies, and the patient’s current problem list are to be reviewed and incorporated as appropriate.
Provider to Patient Exchange (40 points)
The Provider to Patient Exchange measure requires patients have timely access to their health information. The measure is the highest point value measure of the group at 40 points. It requires that information must be available for view, download, and transmission to a third-party. Patients are to have the ability to access their information using an application API of their choice. (Based on 2015 Edition CEHRT technology.)
Public Health and Clinical Data Exchange (10 points)
This objective has six (6) associated measures. Of which an organization needs to select two to report on. Available options are:
- Syndromic Surveillance Reporting
- Immunization Registry Reporting
- Electronic Case Reporting
- Public Health Registry Reporting
- Clinical Data Registry Reporting
- Electronic Reportable Laboratory Result Reporting
The reporting period for Objective Measures is a self-selected 90-day continuous period within the 2020 calendar year. (The 90-day period is a minimum reporting length - organizations may choose a longer period if they wish.)
Report on 4 Clinical Quality Measures (CQMs)
EHs/CAHs are required to report on four (4) CQMs selected from eight (8) available options. (This list of options has been reduced from the previous 16.) The CQM reporting period is one (1) self-selected quarter within the 2020 calendar year. Organizations may submit for this requirement using any combination of QRDA-1 files (Quality Reporting Document Architecture), case threshold exceptions, or zero-denominator declarations. Measures available for selection are:
- ED-2 (CMS111v8) Median Admit Decision Time to ED Departure Time for Admitted Patients
- PC-05 (CMS9v8) Exclusive Breast Milk Feeding
- STK-2 (CMS104v8) Discharged on Antithrombotic Therapy
- STK-3 (CMS71v9) Anticoagulation Therapy for Atrial Fibrillation/Flutter
- STK-5 (CMS72v8) Antithrombotic Therapy by End of Hospital Day 2
- STK-6 (CMS105v8) Discharged on Statin Medication
- VTE-1 (CMS108v8) Venous Thromboembolism Prophylaxis
- VTE-2 (CMS190v8) Intensive Care Unit Venous Thromboembolism Prophylaxis
For more information on CQMs please refer to the eCQI Resource Center.
Attestation Only Requirements
In addition to the Objective and Clinical Quality Measures, EHs/CAHs are also required to submit attestations for the following:
- Security Risk Analysis
- Prevention of Information blocking
- ONC Direct Review
Reporting Exception Requests
In the event that an EH or CAH is unable to report may submit an exception request for one of the following reasons:
- Using decertified EHR technology
- Insufficient Internet connectivity
- Extreme and uncontrollable circumstances
- Lack of control over the availability of CEHRT

